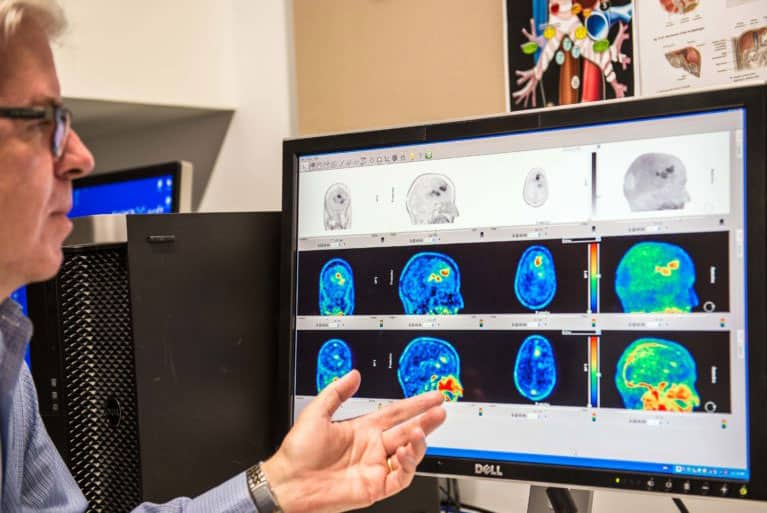


The Australasian Radiopharmaceutical Trials network is a collaborative network incorporating medical specialists, technologists, scientists and researchers from the field of Nuclear Medicine and Molecular Imaging with a shared interest in multicentre clinical trials that utilise radiopharmaceuticals for imaging or therapy.
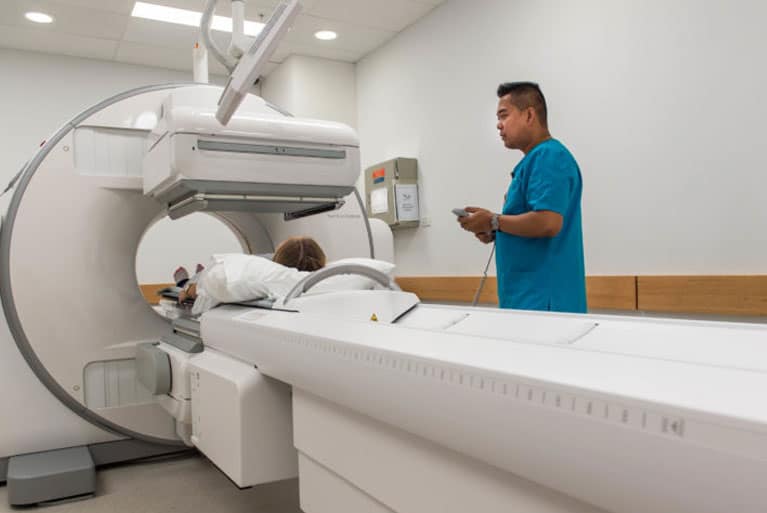
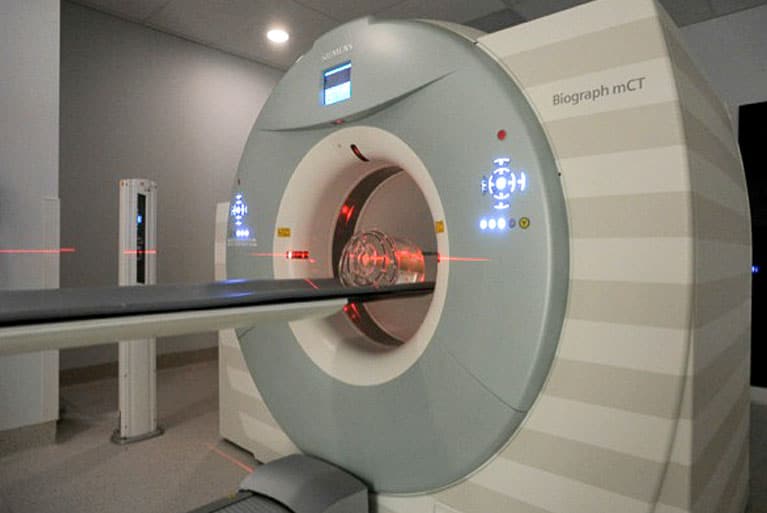
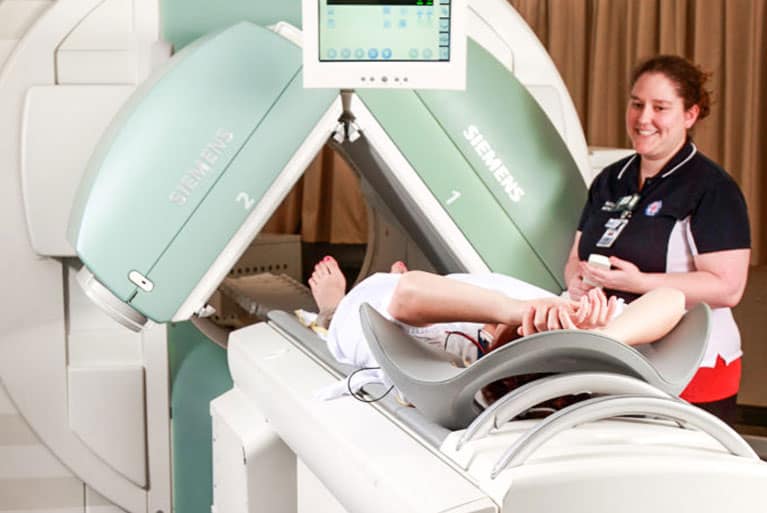
Mission Statement
To promote and facilitate innovative collaborative clinical research utilising radiopharmaceuticals for imaging or therapy.
Vision
-
to develop a network of radiopharmaceutical imaging and therapy sites in Australasia with validated capabilities;
-
to harmonise imaging protocols for research;
-
to facilitate linkages with other clinical trials networks, the pharma industry and funding agencies for multicentre clinical trials;
-
to support multicentre clinical trials with radiopharmaceutical for imaging or therapy, including facilitating data collection, analysis and data management;
-
to promote collaboration in clinical trials and outcome-based research with radiopharmaceuticals
Values
Quality – capitalising on our diverse expertise to deliver excellent outcomes
Innovation – implementing novel ideas to improve health care
Integrity – demonstrating a high level of professionalism in our endeavours
Leadership – being a strong role model for the conduct of high-quality, collaborative and innovative clinical trials, providing mentorship and training for the next generation of clinical researchers
Collaboration – developing new, and strengthening existing, relationships to create unique opportunities.
Mission Statement
To promote and facilitate innovative collaborative clinical research utilising radiopharmaceuticals for imaging or therapy.
Vision
-
to develop a network of radiopharmaceutical imaging and therapy sites in Australasia with validated capabilities;
-
to harmonise imaging protocols for research;
-
to facilitate linkages with other clinical trials networks, the pharma industry and funding agencies for multicentre clinical trials;
-
to support multicentre clinical trials with radiopharmaceuticals for imaging or therapy, including facilitating data collection, analysis and data management;
-
to promote collaboration in clinical trials and outcome-based research with radiopharmaceuticals.



